


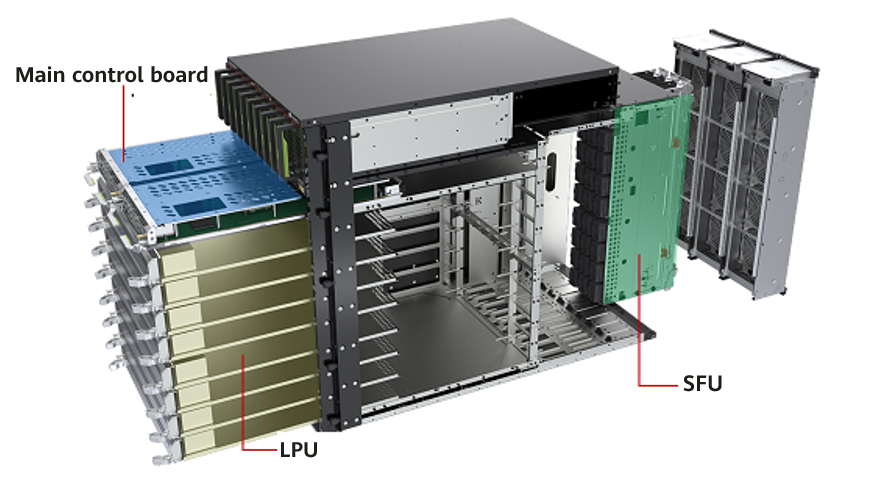
























The United States Food and Drug Administration (FDA) awarded the breakthrough device designation to a Scottish medtech company Altair Medical the FDA's for a wearable biosensor technology that detects a potential opioid overdose death. The wearable tech, named Respmeter, has algorithms designed to monitor respiratory patterns and detect when a wearer shows a sign of opioid-induced respiratory depression. Then, the device will notify emergency responders to administer overdose antidote naloxone. The FDA breakthrough device designation is reserved for products in development that can more effectively treat or diagnose potentially deadly or irreversibly debilitating diseases or conditions. Awarded companies are able to work closer with the FDA during the development process.
 Горячие метки:
Споры в.org
В интернете вещей
Горячие метки:
Споры в.org
В интернете вещей