






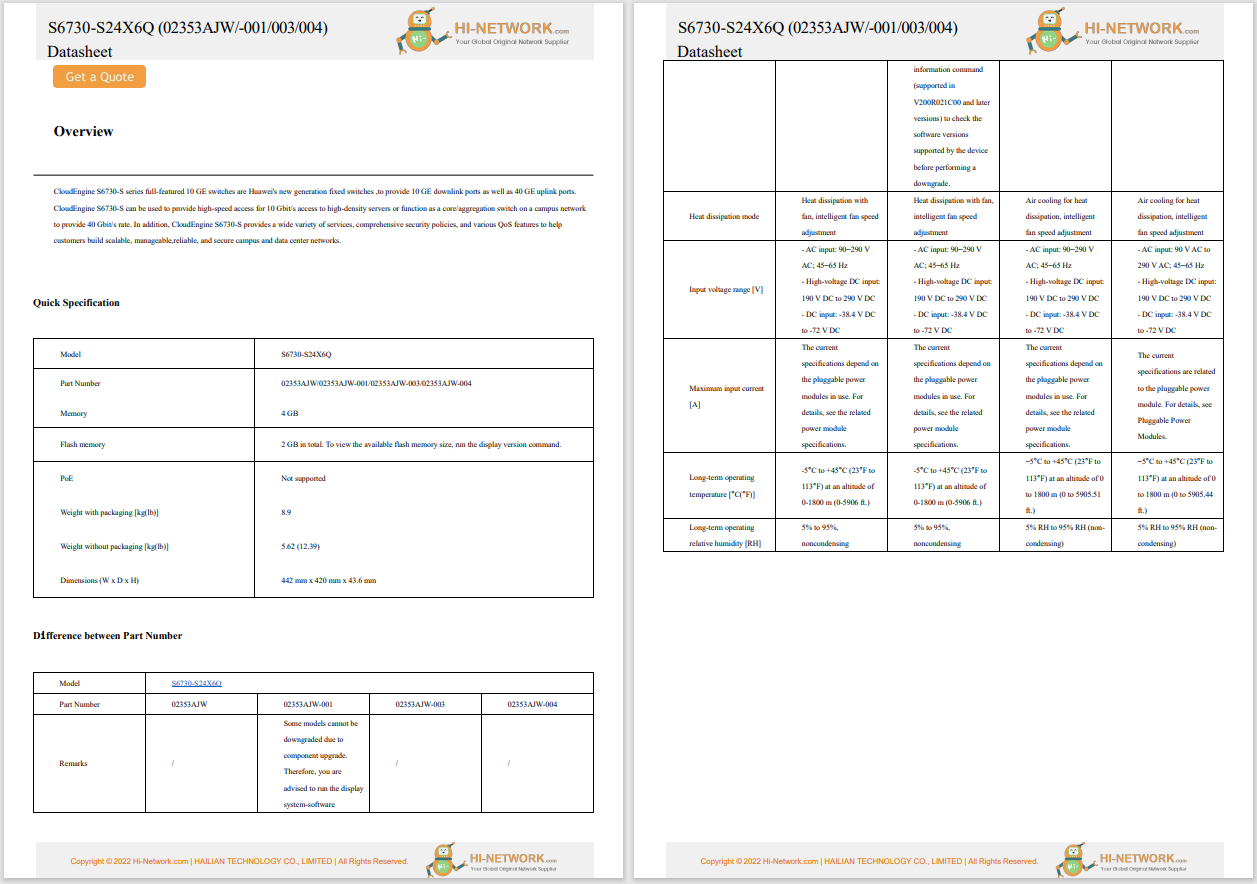
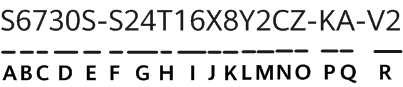


















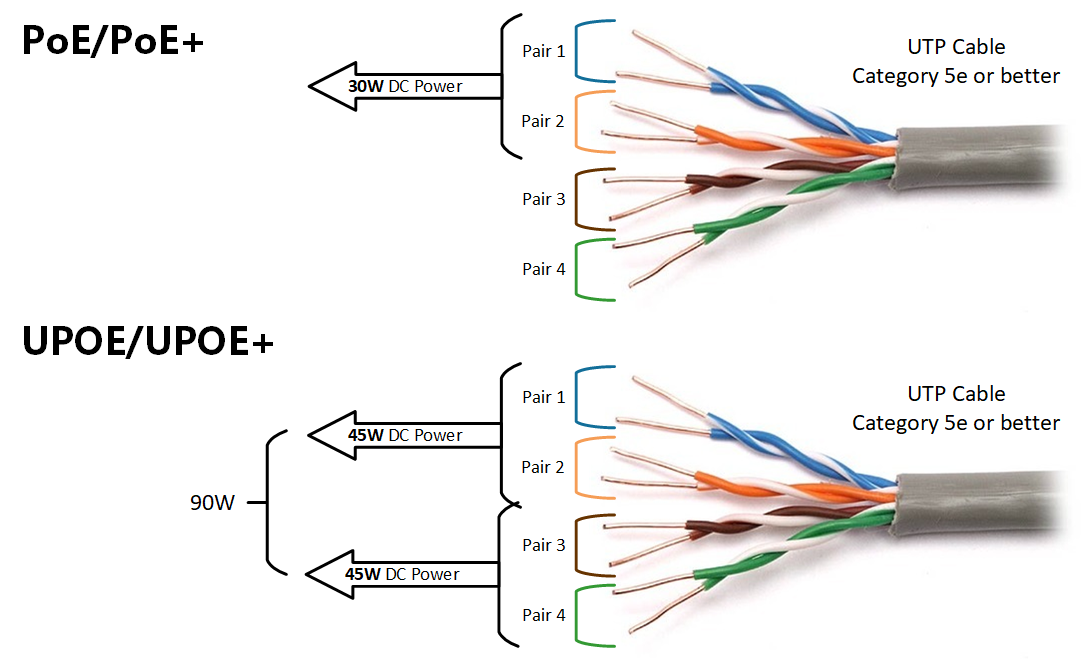
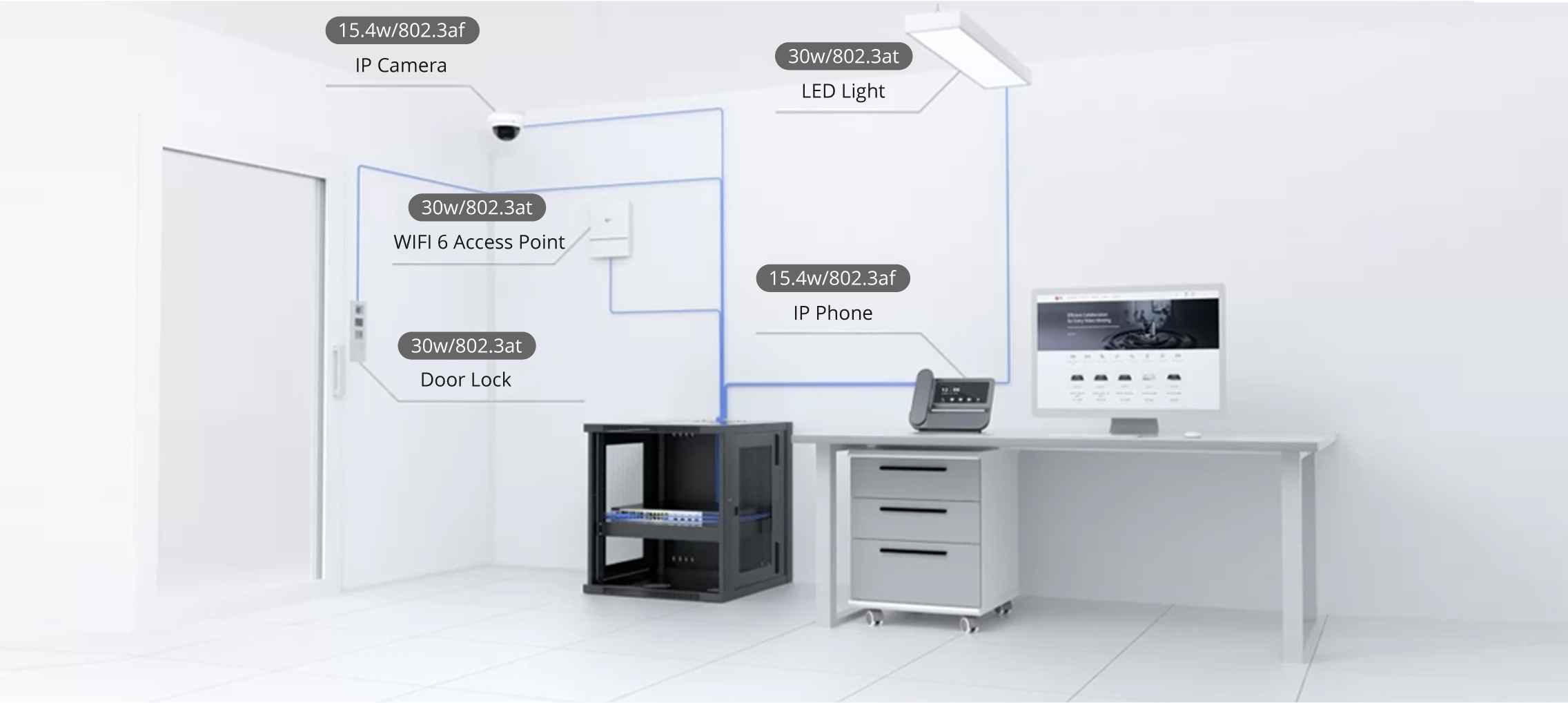



The US Food and Drug Administration (FDA) announced Kevin Fu as its first medical device cybersecurity acting director. Fu will help guide the agency's regulatory strategies regarding medical device cybersecurity issues. He will serve a one-year term as an expert-in-residence at the agency's Center for Devices and Radiological Health (CDRH) and at the Digital Health Centre of Excellence, which launched last fall.
 Горячие метки:
Безопасность в сети
В интернете вещей
Цифровое здравоохранение: применение технологий
Горячие метки:
Безопасность в сети
В интернете вещей
Цифровое здравоохранение: применение технологий
Зарегистрируйтесь по электронной почте сейчас для еженедельной акции акции
100% free, Unsubscribe any time!
Add 1: Room 605 6/F FA YUEN Commercial Building, 75-77 FA YUEN Street, Mongkok KL, HongKong Add 2: Room 405, Building E, MeiDu Building, Gong Shu District, Hangzhou City, Zhejiang Province, China
Whatsapp/ тел: +8618057156223 * телефон: *: 0086 571 86729517 Tel in HK: 00852 66181601
Электронная почта: [email protected]
 English
English Pусский
Pусский Français
Français Español
Español Português
Português